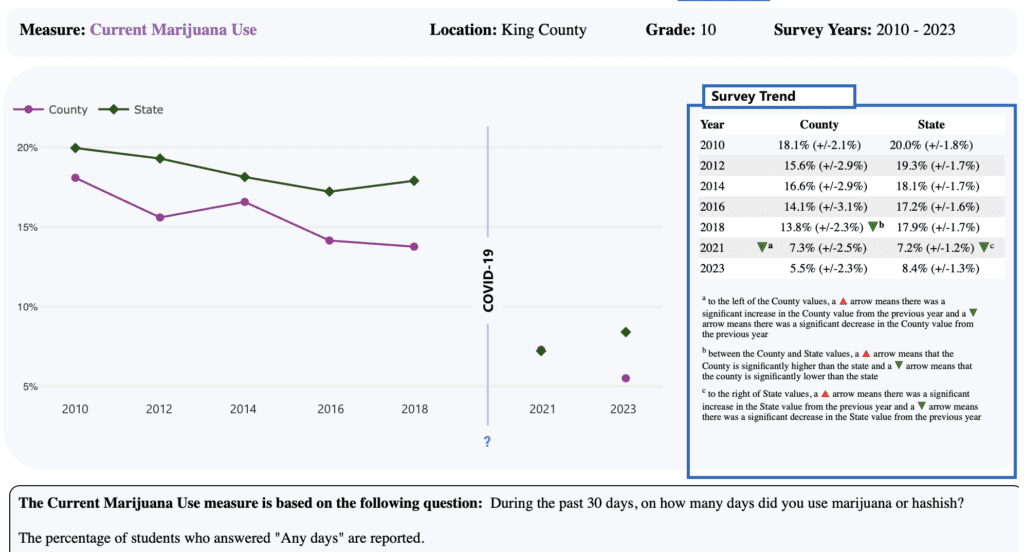
‘No Evidence’ That Marijuana Legalization For Adults Increases Youth Cannabis Use, New Research Published By American Medical Association Finds
Science & Health Archives – Marijuana Moment Read More [[{“value”:” Authors of a new research letter published by the Journal of the American Medical Association (JAMA) on Wednesday said there’s no evidence that states’ adoption of laws to legalize and regulate marijuana for adults have led to an increase in youth use of cannabis. To […]